12th Chemistry Exam questions of Chapter 10

12th chemistry Chapter 10 – Haloalkanes and Haloarenes
Welcome, 12th chemistry exam Important question based on CBSE board curriculum and corresponding to the recent 12 class Chemistry syllabus in text questions.
By rehearsing these Class 12 significant inquiries, understudies will actually want to rapidly survey every one of the thoughts shrouded in the part and plan for the Class 12 Yearly assessments as well as other selection tests like NEET and JEE.
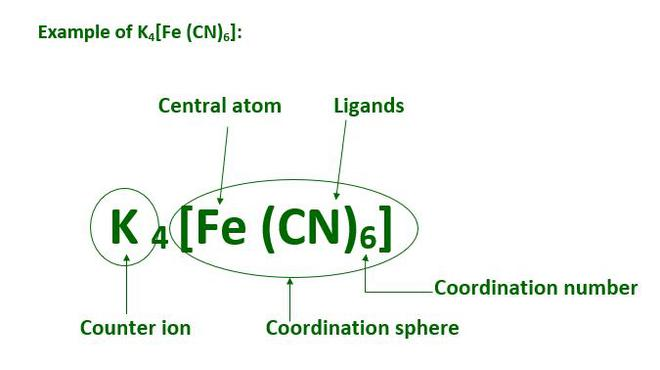
Coordination entity:
A central metal atom/ion bonded to fixed number of oppositely charged ions or neutral molecules. Examples include [Ni(CO)4],[CoCl3(NH3)3].
Ligand:
An atom or molecule or ion capable of donating a pair of electrons to the central metal or ion and forms a coordinate bond with it. Examples include chloride ions, water and ammonia.
Coordination number:
The total number of ligands in a coordination compound attached to the central metal atom or ion. For example, in [Ni(CO)4], the coordination number of Ni is 4. In CoCl3(NH3)3, the coordination number of Co3+ is 6.
Coordination polyhedron:
The spatial arrangement of ligand atoms which are directly attached to the central atom. For example, [Co(NH3)6]3+ has octahedral geometry and Ni(CO)4 has tetrahedral geometry.
homoleptic :
Metal is bound to only one kind of donor groups. For example [Ni(NH3)6]2+,[Co(NH3)6]3+.
heteroleptic :
Metal is bound to more than one kind of donor groups. For example [CoCl3(NH3)3],[NiCl2(H2O)4].
Which of the compounds will react faster in SN1 reaction with the “OH” ion? CH3-CH2-Cl or C6H5-CH2-Cl. (Chemistry book)
During the SN1 reaction’s rate-determining stage, a carbocation intermediate is first produced. In benzyl chloride, the benzyl carbocation is resonance stabilized.
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts. But why does the preparation of aryl iodides require the presence of an oxidising agent?(Chemistry book)
Iodination reactions occur reversibly in nature. To continue the reaction in the forward direction, oxidation removes the HI produced during iodination. HIO4 is used as an oxidizing agent. Iodination reactions occur reversibly in nature. To continue the reaction in the forward direction, oxidation removes the HI generated during iodination. HIO4 is used as an oxidizing agent.
Haloarenes are less reactive than haloalkanes and haloarenes. Explain.(Chemistry book)
The carbon-halogen bond’s polarity: As we all know, reactivity rises with polarity. Because the C-X bond in haloarenes has a lower dipole moment than that of the haloalkane (for instance, the dipole moment for C-X in haloalkane is 2.0-2.2D while the dipole moment for chlorobenzene is 1.7D), the polarity of a haloalkane increases with its polarity.
Haloarenes are found in the sp2 hybridization state, meanwhile haloalkanes are found in the sp3 hybridization state.. The bond formed by the haloarenes is stronger than the haloalkane bond because the bond length in the sp2 hybridization is shorter than in the sp3 hybridization. haloarenes are less reactive as a result.
Resonance stabilization occurs in haloarenes when the electron of the benzene ring combines with the electron pair of the halogen. The C-X bond has a partial double bond character due to the resonance hybrid nature of haloarenes, which makes the haloarene less reactive and more stable.
Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.(Chemistry book)
Species without electrons are known as Lewis acids. They are the ones that trigger heterolytic fission in halogen molecules. The creation of an electrophile is the role of Lewis acid. The electron-rich benzene ring is attacked by the electrophile, which then forms aryl bromides and chlorides. The formation of an electrophile is the role of Lewis acid. The electron-rich benzene ring is attacked by the electrophile, and the result forms aryl bromides and chlorides.
By electrophilically substituting arenes with bromine or chlorine, respectively, aryl bromides and chlorides can be created in the presence of Lewis acid catalysts (iron or iron chloride).
Why does iodoform have appreciable antiseptic properties?(Chemistry book)
Iodine is produced when iodoform (CHI3) comes into contact with skin, and this is what provides the substance its antiseptic properties. Iodoform releases I2 when it comes into contact with skin. It is not iodoform per se that has antiseptic characteristics; instead, it is the release of I2.
Why is the solubility of haloalkanes in water very low?(Chemistry book)
Haloalkanes are only weakly soluble in water because energy is needed to dissolve them due to the attraction between the haloalkane molecules and the breaking of hydrogen bonds between water molecules. Conversely, because the new attractions between the haloalkane and the water molecules are weaker than the original hydrogen bonds in water, they release less energy.
Write down the structure and IUPAC name for neopentyl bromide.(Chemistry book)
IUPAC name ⇒ 1-bromo-2,2-dimethylpropane
Common name ⇒ neo-pentylbromide
A hydrocarbon of molecular mass 72 g mol/l gives a single monochloro derivative and two dichloro derivatives on photo chlorination. Give the structure of the hydrocarbon.(Chemistry book)
Molecular mass of hydrocarbon = 72
The molecular formula for an alkane, or saturated hydrocarbon, is CnH2n+2.
Consequently, the molecular formula of hydrocarbon is C5H12
The isomer of pentane that produces a single monochloro derivative should contain all 12 hydrogen atoms equivalent to pentane’s molecular mass of 72g mol−1.
Why can aryl halides not be prepared by reaction of phenol with HCl in the presence of ZnCl2?(Chemistry book)
A carbocation is produced when an alkyl halide is formed, and it responses with HCl to generate a different alkyl halide.
However, phenyl carbocation formation, which is impossible because it is a highly unstable structure that never occurs in its free state, is required for aryl halide to react with HCl in the presence of ZnCl2. Consequently, no aryl halide is produced.
Why is it necessary to avoid even traces of moisture during the use of a Grignard reagent?(Chemistry book)
Grignard reagents are materials that react rapidly. Hydrocarbons are generated when they react with any source of protons. Even water has enough acidity in it to change it into a suitable hydrocarbon. Due to Grignard reagents are extremely reactive, it is imperative to avoid even the smallest amount of moisture when using them. Grignard reagents are very reactive and produce corresponding hydrocarbons when they react with water.
RMgX + H2O → RH + Mg(OH)X.
Allyl chloride is hydrolysed more readily than n-propyl chloride. Why?(Chemistry book)
Since resonance stabilizes the carbocation formed during hydrolysis, allyl chloride is highly reactive while n-propyl chloride lacks this stabilization.
Allyl chloride is more easily hydrolyzed than n-propyl chloride because the latter does not go through ionization to form n-propyl carbocation.
Discuss the nature of C-X bond in the haloarenes.(Chemistry book)
In haloarenes, the carbon of benzene is bonded to the halogen. Halogen has a higher electronegativity than the sp2 hybridized carbon of the benzene ring. The C-X bond is therefore a polar bond. In addition, the lone pair of electrons in the halogen atom are involved in the resonance of the benzene ring. This C-X bond now has a partial bond character as a result.
The C-X bond of haloarenes is less polar than that of haloalkanes. This is supported by the fact that the dipole moment of CH3CI (= 1.83 D) is slightly higher than that of chlorobenzene (= 1.69 D).
Cyanide ion acts as an ambident nucleophile. From which end does it act as a stronger nucleophile in an aqueous medium? Give reason for your answer.(Chemistry book)
Because it leads to in the formation of a C-C bond, which is more stable than a C-N bond, it acts as a bigger nucleophile from the carbon end. Since the cyanide ion (C= N) can react via nitrogen, it is a common nucleophile. Due to the C-C bond is more powerful than the C-N bond, alkyl cyanide is created mainly by cyanide ionizing carbon.
Check our 12th Chemistry exam solution of chapter 8 click here.
Check our 12th Chemistry exam solution of chapter 9 click here.
Want some good health click here.



Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?