12th chemistry Chapter 9 – coordination compounds
Welcome, 12th chemistry exam Important question based on CBSE board curriculum and corresponding to the recent 12 class Chemistry syllabus in text questions.
By rehearsing these Class 12 significant inquiries, understudies will actually want to rapidly survey every one of the thoughts shrouded in the part and plan for the Class 12 Yearly assessments as well as other selection tests like NEET and JEE.
Write the formulas for the following coordination compounds:
(a) Tetraammineaquachloridocobalt(III) chloride
Ans: [Co(NH3 ) 4 (H2O)Cl]Cl2
(b) Potassium tetrahydroxidozincate(II)
Ans:K2 [Zn(OH)4 ]
(c) Potassium trioxalatoaluminate(III)
Ans:K3 [Al(C2O4 ) 3 ]
(d) Dichloridobis(ethane-1,2-diamine)cobalt(III)
Ans:) [CoCl2 (en)2 ]+
(e) Tetracarbonylnickel(0)
Ans:[Ni(CO)4 ]
Write the IUPAC names of the following coordination compounds:
(a) [Pt(NH3 )2Cl(NO2 )]
Ans: Diamminechloridonitrito-N-platinum(II)
(b) K3 [Cr(C2O4 )3 ]
Ans: Potassium trioxalatochromate(III)
(c) [CoCl2 (en)2 ]Cl
Ans: Dichloridobis(ethane-1,2-diamine)cobalt(III) chloride
(d) [Co(NH3 )5 (CO3 )]Cl
Ans: Pentaamminecarbonatocobalt(III) chloride
(e) Hg[Co(SCN)4 ]
Ans: Mercury (I) tetrathiocyanato-S-cobaltate(III)
Why is geometrical isomerism not possible in tetrahedral complexes
having two different types of unidentate ligands coordinated with
the central metal ion?(Chemistry book)
1) For geometrical isomerism, the relative position of unidentate ligands attached to central metal atom shall be unequal.
2)In case of tetrahedral complex, the positons of the unidentate ligands are identical with respect to each other and hence show optical isomerism and not geometric.
Tetrahedral complexes do not show geometrical isomerism because
the relative positions of the unidentate ligands attached to the central
metal atom are the same with respect to each other.(Chemistry book)
Let us assume a metal ion M (n+) surrounded by four ligands a,b,c&d making a complex. Since metal ion surrounded by four ligands it can adapt either tetrahedral shape or square planar shape. Between these two in square planar geometry four ligands and the metal ion are in same plane (like children are sitting in floor (it is a plane) or upstairs or terrace. These are different planes)
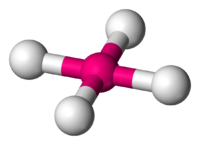
So in square planar geometry, two ligands will be cis to each other and the third ligand will be farther away or opposite corner or trans to each other. So likewise we can tell cis or trans with respect to ligands a,b,c & d.
Whereas in tetrahedral molecule central metal ion and four ligands are in different planes. (Like people are sitting on staircases, each step is in different plane)
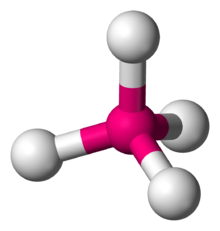
So if you choose a ligand, other three will be adjacent to the reference ligand. So there will not be any geometric isomers even though metal ion contains four different ligands. But it will have optical isomers which is called enantiomer.
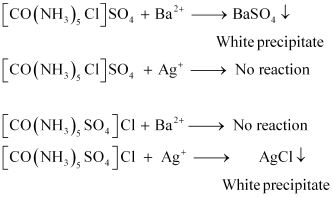
Give evidence that [Co(NH3 )5Cl]SO4 and [Co(NH3 )5 (SO4 )]Cl are ionisation isomers.(Chemistry book)
The spin only magnetic moment of [MnBr4]2– is 5.9 BM. Predict the
geometry of the complex ion ?(Chemistry book)
Since the coordination number of Mn2+ ion in the complex ion is 4, it will be either tetrahedral (sp3 hybridisation) or square planar (dsp2 hybridisation). But the fact that the magnetic moment of the complex ion is 5.9 BM, it should be tetrahedral in shape rather than square planar because of the presence of five unpaired electrons in the d orbitals.
Explain on the basis of valence bond theory that [Ni(CN)4 ] 2– ion with square planar structure is diamagnetic and the [NiCl4 ] 2– ion with tetrahedral geometry is paramagnetic.(Chemistry book)
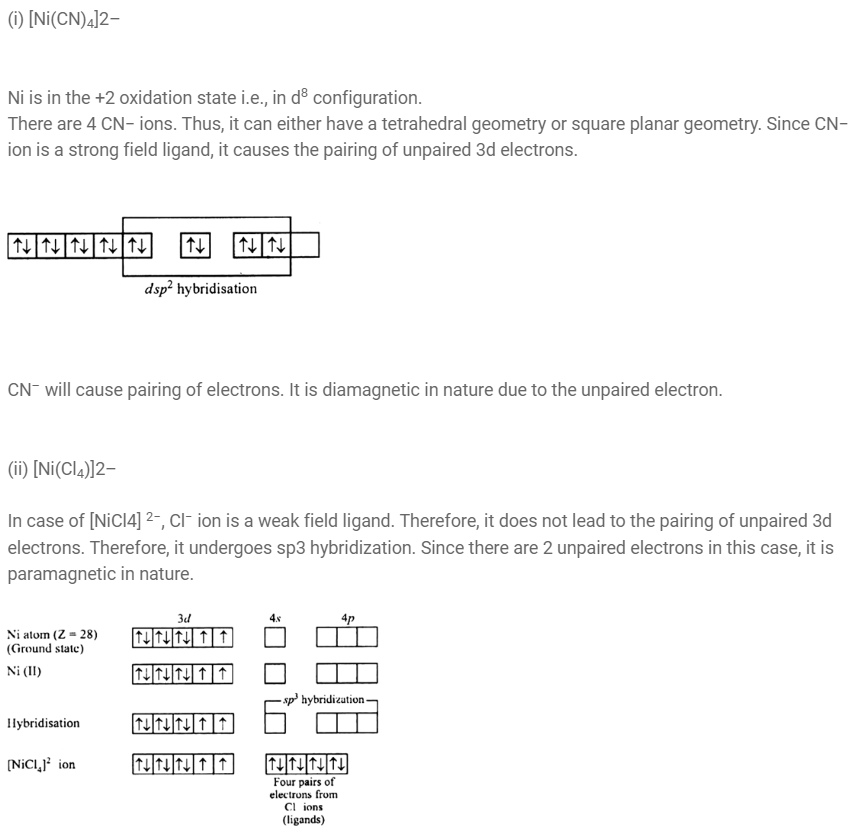
[NiCl4 ] 2– is paramagnetic while [Ni(CO)4 ] is diamagnetic though both are tetrahedral. Why?
In [NiCl4]2- Ni is in+2 oxidation state with the configuration 3d8 4so.Cl- ion being weak ligand it cannot pair up the electrons in 3dorbitals. Hence it is paramagnetic. In [Ni CO4] Ni is in zero oxidation with the configuration 3d8 4s2. In the presence of CO ligand the 4s electrons shift to 3d to pair up 3d electrons.
[Fe(H2O)6 ] 3+ is strongly paramagnetic whereas [Fe(CN)6 ] 3– is weakly paramagnetic. Explain
The correct option is A Both Assertion and Reason are correct and Reason is the correct explanation for Assertion
[Fe(CN)6]3−⇒C\bar N\Rightarrow$ Strong field ligand.
Fe3+=[Ar]3d5(Refer o image 01)
1. 1 unpaired electrons.
2. Weakly paramagnetic
[Fe(H2O)6]3+⇒H2O⇒ Weak field ligand.
⇒Fe3+⇒[Ar]3d5 (Refer o image 02)
5 unpaired electrons.
[Fe(H2O)6]2+=Strong paramagnetic.
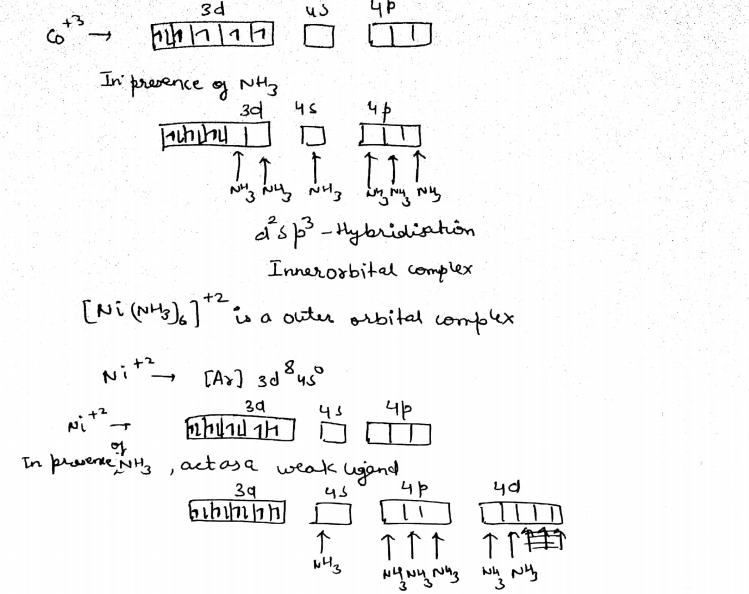
Explain [Co(NH3 )6 ] 3+ is an inner orbital complex whereas [Ni(NH3 )6 ] 2+ is an outer orbital complex.
[CO(NH3)6]3+ is inner orbital complex
CO→[Ar]3d74s2
CO+3→[Ar]3d64s0
With +3 oxidation state NH3 act as a strong ligand.
Predict the number of unpaired electrons in the square planar [Pt(CN)4 ] 2– ion.
no unpaired electrons
CN− being a strong field ligand causes the pairing of unpaired electrons. Hence, there are no unpaired electrons in.
The hexaquo manganese(II) ion contains five unpaired electrons, while the
hexacyanoion contains only one unpaired electron. Explain using Crystal
Field Theory
Mn(II) has an electronic configuration of 3d54s0. In the case of hexaaquomanganese (II) ion the ligand attached to metal is water which is a weak field ligand and is not able to pair up the electrons of 3d subshell and the configuration is t2g3 and eg2. whereas in the case of hexacyano ion the CN− is a strong field ligand and it causes pairing of electrons in the d orbitals and hence thee exist only one unpaired electron in t2g5eg0.
Explain the bonding in coordination compounds in terms of Werner’s postulates.
Werner’s theory is the first theory to explain the nature of bonding in coordination compounds. The main postulates of this theory are: (i) Two types of valencies, primary and secondary valencies, are present in coordinated compound metals. (iii) The primary valence is equivalent to the metal ion oxidation number.
Explain with two examples each of the following: coordination entity, ligand,
coordination number, coordination polyhedron, homoleptic and heteroleptic.
Coordination entity:
A central metal atom/ion bonded to fixed number of oppositely charged ions or neutral molecules. Examples include [Ni(CO)4],[CoCl3(NH3)3].
Ligand:
An atom or molecule or ion capable of donating a pair of electrons to the central metal or ion and forms a coordinate bond with it. Examples include chloride ions, water and ammonia.
Coordination number:
The total number of ligands in a coordination compound attached to the central metal atom or ion. For example, in [Ni(CO)4], the coordination number of Ni is 4. In CoCl3(NH3)3, the coordination number of Co3+ is 6.
Coordination polyhedron:
The spatial arrangement of ligand atoms which are directly attached to the central atom. For example, [Co(NH3)6]3+ has octahedral geometry and Ni(CO)4 has tetrahedral geometry.
homoleptic :
Metal is bound to only one kind of donor groups. For example [Ni(NH3)6]2+,[Co(NH3)6]3+.
heteroleptic :
Metal is bound to more than one kind of donor groups. For example [CoCl3(NH3)3],[NiCl2(H2O)4].
Check our 12th Chemistry exam solution of chapter 1 click here.
Check our 12th Chemistry exam solution of chapter 2 click here.
Check our 12th Chemistry exam solution of chapter 3 click here.
Check our 12th Chemistry exam solution of chapter 4 click here.
Check our 12th Chemistry exam solution of chapter 5 click here.
Check our 12th Chemistry exam solution of chapter 6 click here.
Check our 12th Chemistry exam solution of chapter 7 click here.
Check our 12th Chemistry exam solution of chapter 8 click here.
Want some good health click here.
