12th chemistry Chapter 6: General Principles and Processes of Isolation of Elements
Welcome, 12th chemistry exam Important question based on CBSE board curriculum and corresponding to the recent 12 class Chemistry syllabus in text questions.
By rehearsing these Class 12 significant inquiries, understudies will actually want to rapidly survey every one of the thoughts shrouded in the part and plan for the Class 12 Yearly assessments as well as other selection tests like NEET and JEE.
Check our 12th Chemistry exam solution of chapter 1 click here.
Check our 12th Chemistry exam solution of chapter 2 click here.
Check our 12th Chemistry exam solution of chapter 3 click here.
Check our 12th Chemistry exam solution of chapter 4 click here.
Check our 12th Chemistry exam solution of chapter 5 click here.
At a temperature above 1073K, coke can reduce FeO to Fe. How can you justify this reduction with the Ellingham diagram?(Chemistry book)
The Ellingham diagram indicates that at temperatures higher than 1073 K, ΔG(C, CO) < ΔG(Fe, FeO). Cocaine can thus convert FeO to Fe.
Why is an external emf of more than 2.2V required for the extraction of Cl₂ from brine?(Chemistry book)
For the reaction:
2Cl− (aq) + 2H2O (l) → 2OH− (aq) + H2 (g) + Cl2 (g),
For the given reaction,the value of ΔG∘ is + 422kJ.
Using the equation ΔG∘ = − nFE∘ , the value of E∘ comes out to be -2.2 V.
Therefore, extracting Cl2 from brine will require an external emf of greater than 2.2 V.
How is copper extracted from low grade copper ores?(Chemistry book)
Using hydrometallurgy, copper is extracted from low-grade copper ore. Bacteria are used to leach the ore out for this purpose. Cu2+-containing solution is treated with H2 and scrap iron.
Cu2+ (aq) + H2 (g) → Cu (s) + 2H+ (aq)
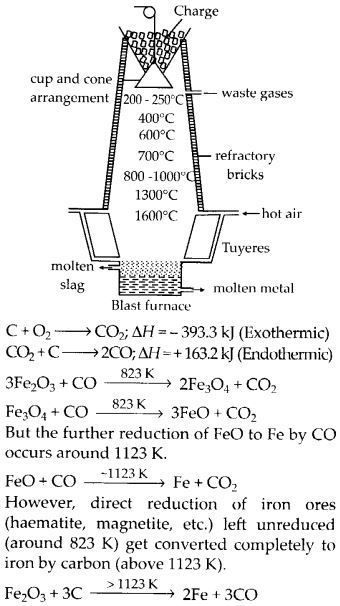
Wrought iron is the purest form of iron. Write a reaction used for the preparation of wrought iron from cast iron. How can the impurities of sulphur, silicon and phosphorus be removed from cast iron?(Chemistry book)
The purest type of commercial iron is wrought iron. It is made from cast iron by oxidizing the impurities in a haematite-lined reverberatory furnace.
Fe2O3 + 3C → 2Fe + 3CO
To eliminate the phosphorous, silicon, and sulphur impurities, limestone is added as a flux. They combine to form an easily removed slag. Rolls are used to extract the metal from the slag.
The purest form of iron is prepared by oxidising impurities from cast iron in a reverberatory furnace. Which iron ore is used to line the furnace? Explain by giving a reaction.(Chemistry book)
By oxidizing the impurities in a reverberatory furnace lined with haematite, wrought iron is produced from cast iron. Carbon is oxidized to carbon monoxide by haematite.
Fe2O3 + 3C → 2Fe + 3CO
To eliminate the phosphorous, silicon, and sulphur impurities, limestone is added as a flux. They combine to form an easily removed slag. Rolls are used to extract the metal from the slag.
How Do We Separate Two Sulphide Ores By Froth Floatation Method?(Chemistry book)
The ratio of water to oil needs to be changed in order to separate two sulphide ores. Another way to accomplish this is by taking depressants like NaCN. During the process, NaCN is added if an ore contains PbS and ZnS. ZnS combines with NaCN to form a complex that keeps it from foaming. PbS follows into the foam as well, which is how it separates.
The mixture of compounds A and B is passed through a column of Al2O3 by using alcohol as eluent. Compound A is eluted in preference to compound B. Which of the compounds A or B, is more readily adsorbed on the column?(Chemistry book)
Given that compound A elutes more readily than compound B. On the column, compound B is adsorbed more easily.
Although carbon and hydrogen are better reducing agents, they are not used to reduce metallic oxides at high temperatures. Why?(Chemistry book)
Since carbon and hydrogen react at high temperatures with metals to form carbides and hydrides, respectively, they are not used.
Why is sulphide ore of copper heated in a furnace after mixing with silica?(Chemistry book)
Slag, or iron silicate, is created when iron oxide is present as an impurity in copper sulphide ore, and copper is produced as copper matte.
FeO + SiO2 → FeSiO3
Which method is used for refining Zr and Ti? Explain with equations.(Chemistry book)
Zinc and titanium are refined using the Van Arkel method. This process involves heating crude metal while using iodine.
Two factors are usually taken into account in order to take the necessary safety measures.
conformity of the electrodes.
metal’s produced reactivity.
Why are sulphide ores converted to oxide before reduction?(Chemistry book)
Because oxides reduce more quickly than sulphides do, ores rich in sulphides are first converted to oxides.
What should be the considerations during the extraction of metals by the electrochemical method?(Chemistry book)
While using the electrochemical near to extract metals, the following factors need to be taken into account:
- Reactivity of the generated metal
- Flux addition to make molten mass conducting
- suitable materials must be used to make electrodes
What is the role of flux in metallurgical processes?(Chemistry book)
Any material added during the smelting of ores to increase fluidity and eliminate impurities in the form of slag is known as flux.
Role of flux in the metallurgical process:
By combining it with flux, the gangue is eliminated. Slag formation results as a result.
Give two requirements for vapour phase refining.(Chemistry book)
Two requirements for vapour phase refining are
- Decomposition should be used to recover the volatile compound as soon as possible.
- With an accessible reagent, the metal ought to combine to form a volatile compound.
Write the Chemical reactions taking place in different zones in the blast furnace for the extraction of iron from its ore.(Chemistry book)
The following chemical processes are occurring in various blast furnace zones in order to extract iron from its ore:
3Fe2O3+CO→2Fe3O4+CO2
Fe3O4+4CO→3Fe+4CO2 (in the temperature range of K500 – 800K)
Fe2O3+CO→2Fe+CO2
C+CO2→2CO
FeO+CO→Fe+CO2 (in the temperature range of 900 – 1500K)
Why is the reduction of a metal oxide easier if metal formed is in liquid state at the temperature of reduction?(Chemistry book)
A metal’s entropy is higher in its liquid state than in its solid state, which causes the Gibbs free energy to decrease and the reduction process to proceed more quickly.
Copper can be extracted by hydrometallurgy but not zinc. Explain why?(Chemistry book)
Hydrometallurgy can be used to extract copper, but not zinc because of zinc’s lower reduction potential than copper’s. in order for zinc to reduce copper from its solution.
Although aluminium is above hydrogen in the electrochemical series, it is stable in air and water. Why? (Chemistry book)
Due to the production of the inert oxide Al2O3, aluminium is stable in air and water despite being above hydrogen in the electrochemical series.
A sample of galena is contaminated with zinc blend. Name one chemical which can be used to concentrate galena selectively by froth floatation method.(Chemistry book)
Sodium cyanide (NaCN) is the chemical that is used, and it can be concentrated using the froth floatation method.
Want some good health click here.
