12th Chemistry Chapter 7 – P – Block Elements
Welcome, 12th chemistry exam Important question based on CBSE board curriculum and corresponding to the recent 12 class Chemistry syllabus in text questions.
By rehearsing these Class 12 significant inquiries, understudies will actually want to rapidly survey every one of the thoughts shrouded in the part and plan for the Class 12 Yearly assessments as well as other selection tests like NEET and JEE.
PH3 forms bubbles when passed slowly in water, but NH3 dissolves. Explain why?(Chemistry book)
In contrast to NH3, which dissolves because it is soluble in water and can form hydrogen bonds with it, PH3 produces bubbles because it is insoluble in water.
In the preparation of H2SO4 by Contact Process, why is SO3 not absorbed directly in water to form H2SO4 ?(Chemistry book)
The reason why SO3 does not readily condense into a dense fog of sulphuric acid in water is because SO3 is not directly absorbed in water to form H2SO4.
In PCI5, phosphorus is in a sp3d hybridised state, but its five bonds are not equivalent. Justify your answer with reason.(Chemistry book)
SO3 isn’t The structure of PCI5 is trigonal bipyramidal. It consists of two equivalent axial bonds and three equivalent equatorial bonds. Because there is more repulsion between the three equatorial bonds, axial bonds must be larger than equatorial bonds in order to overcome it directly absorbed in water to produce H2SO4 since SO3 creates a thick, difficult-to-condense sulphuric acid fog.
As a result, two axial P-Cl bonds differ from equatorial bonds and are longer.
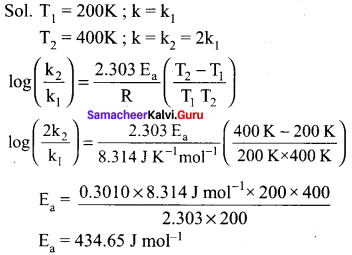
Give reason to explain why CIF3 exists but FCl3 does not exist.(Chemistry book)
Because chlorine’s d orbitals are vacant, it can exhibit an oxidation state of +3. Since fluorine lacks d orbitals, it is unable to display a state of positive oxidation. FCl3 does not exist as a result.
That can also be explained by the idea of atomic size. The chlorine atom’s size is more important. Three large chlorine atoms cannot fit inside a small fluorine atom.
Therefore, CIF3 exists, but FCl3 does not exist.
Why is nitric oxide paramagnetic in a gaseous state, but the solid obtained on cooling it is diamagnetic?(Chemistry book)
NO2 exists as a monomer with one unpaired electron in a gaseous state. In the solid state, it dimerises to N2O4 , so no unpaired electron is left; hence solid form is diamagnetic.
SF6 is known, but SCl6 is not. Why?(Chemistry book)
As a fluorine atom is smaller than a sulphur atom, six F-ions can encircle a sulphur atom. Because of their size, chlorine atoms are an exception to this rule. Therefore, because of interionic repulsion between larger ions, SF6 is known while SCl6 is unknown.
Out of H2O and H2S, which one has a higher bond angle and why?(Chemistry book)
Compared to H2S, H2O has a larger bond angle.
Since oxygen is more electronegative than sulphur, the bond pair electron of the O-H bond will be closer to oxygen, resulting in a larger bond angle in H2O. The bond angle would be more noticeable because of bond-pair repulsion between bond pairs of two O-H bonds.
In the ring test of NO3– ion, Fe2+ ion reduces nitrate ion to nitric oxide, which combines with Fe2+ (aq) ion to form a brown complex. Write the reactions involved in the formation of the brown ring.(Chemistry book)
NO3− + 3Fe2+ + 4H+ → NO + 3Fe3+ + 2H2O
[Fe(H2O)6]2+ + NO → [Fe(H2O)5(NO)]2+ + H2O
Explain why the stability of oxyacids of chlorine increases in the order given below: HCIO < HCIO2 < HCIO3 < HCIO4.(Chemistry book)
Compared to chlorine, oxygen is more electronegative. As a result, as chlorine’s oxygen atom count rises, the negative charge that is present on it disperses from ClO– to ClO4– ions. As a result, ions will become more stable in the sequence listed below.
ClO− < ClO2– < ClO3– < ClO4–
The corresponding acid’s acidic strength increases in the same order as a result of the conjugate base’s increased stability.
HCIO < HCIO2 < HCIO3 < HCIO4.
When white phosphorus and chlorine combine, the resulting substance hydrolyzes in the presence of water. Calculate the mass of HCl obtained by the hydrolysis of the product formed by the reaction of 62 g of white phosphorus with chlorine in the presence of water.(Chemistry book)
P4 + 6Cl2 → 4PCl3
[ PCl3 + 3H2O → H3PO3 + 3HCl ] × 4
P4 + 6Cl2 + 12H2O → 4H3PO3 + 12HCl
1 mol of white phosphorus generates 12 moles of HCl
62 g of white phosphorus has been taken, which is equivalent to 62 / 124 = 1 / 2 mol
Therefore 6 moles of HCl will be formed.
Mass of 6 moles of HCl = 6 × 36.5 = 219.0 g of HCl
Explain why ozone is thermodynamically less stable than oxygen.(Chemistry book)
With the help of oxygen, ozone is thermodynamically unstable because when it breaks down into oxygen, heat flows out (ΔH is negative) and entropy is increased (ΔS is positive). When it comes to its conversion into oxygen, these two effects drastically decrease the Gibbs energy change (ΔG).
Name three oxoacids of nitrogen. Write the disproportionation reaction of that oxoacid of nitrogen in which nitrogen is in +3 oxidation state.(Chemistry book)
The three oxoacids of nitrogen are
(1) Nitrous acid, HNO2
(2) Nitric acid, HNO3
(3 ) Hyponitrous acid, H2N2O2
Nitric acid forms an oxide of nitrogen on reaction with P4O10. Write the reaction involved.(Chemistry book)
When nitric acid and P4O10 combine, metaphosphoric acid (HPO3) and an oxide of nitrogen (N2O5) are produced.
The reaction follows
HNO3 + P4O10 → 4HPO3 + 2N2O5 .
Phosphorus forms several oxoacids. Out of these oxoacids, phosphinic acid has strong reducing property. Write a reaction showing its reducing behaviour.(Chemistry book)
The following reaction between phosphinic acid and silver nitrate illustrates the reducing behaviour of the acid:
4AgNO3 + 2H2O + H3PO2 → 4Ag + 4HNO3 + H3PO4
Give an example to show the effect of nitric acid concentration on the formation of an oxidation product.(Chemistry book)
When nitric acid is reacted with copper metal, concentrated and diluted solutions produce distinct oxidation products.
3Cu + 8HNO3 (dil.) → 3Cu(NO3)2 + 2NO + 4H2O
Cu + 4HNO3 (Conc) → 3Cu(NO3)2 + 2NO2 + 2H2O
On heating, compound A gives a gas B, a constituent of air. When treated with 3mol of hydrogen (H2) in the presence of a catalyst, this gas provides another gas with C, which is basic. Gas C on further oxidation in moist conditions gives a compound D part of acid rain. Identify compounds A to D.(Chemistry book)
Give the necessary equations of all the steps.
Here
A = NH4NO2
B = N2
C = NH3
D = HNO3
The required formulas are:
NH4NO2 → N2 + 2H2O
N2 + 3H2 → 2NH3
4NH3 + 5O2 → 4NO + 6H2O
4NO + O2 → 4NO2
3NO2 + H2O → 2HNO3 + NO
Check our 12th Chemistry exam solution of chapter 1 click here.
Check our 12th Chemistry exam solution of chapter 2 click here.
Check our 12th Chemistry exam solution of chapter 3 click here.
Check our 12th Chemistry exam solution of chapter 4 click here.
Check our 12th Chemistry exam solution of chapter 5 click here.
Check our 12th Chemistry exam solution of chapter 6 click here.
Want some good health click here.
