12th Chemistry Exam questions of Chapter 8

12th chemistry Chapter 8 – D and F Block Elements
Welcome, 12th chemistry exam Important question based on CBSE board curriculum and corresponding to the recent 12 class Chemistry syllabus in text questions.
By rehearsing these Class 12 significant inquiries, understudies will actually want to rapidly survey every one of the thoughts shrouded in the part and plan for the Class 12 Yearly assessments as well as other selection tests like NEET and JEE.
Why is the first ionisation enthalpy of Cr is lower than that of Zn?(Chemistry book)
Compared to Zn, Cr has a lower ionization enthalpy due to its stable configuration. The electron exits the fully filled 4s orbital in the case of zinc. Therefore, compared to chromium, zinc requires more energy to remove an electron.
Why does copper not replace hydrogen from acids?(Chemistry book)
Since copper has a positive E∘ value—that is, it is less reactive than hydrogen, which has an electrode potential of 0.0 V—it does not replace hydrogen in acids. Thus, Cu cannot take the place of hydrogen in acids.
Why are E- values for Mn, Ni and Zn more negative than expected?(Chemistry book)
The metals will oxidize and lose their electrons very quickly. The oxidized species is more stable than the reduced species when the E value is negative. Because their d orbitals are half-filled and fully filled, respectively, Mn2+ (3d5) and Zn2+ (3d10) have stability and would rather remain that way rather than be reduced. First and second ionization enthalpies balance the extremely high negative hydration enthalpy of Ni2+ (3d8).
Out of Cu2Cl2 and CuCl2, which is more stable and why?(Chemistry book)
Compared to Cu2Cl2, CuCl2 is more stable. Because Cu2+ (aq) has a far more negative ΔhydH∘ than Cu+ (aq), it is more stable than Cu+ (aq).
Transition elements show high melting points. Why?
The increased participation of (n-1)d electrons in addition to ns electrons in the interatomic metallic bonding is the cause of the high melting points of transition metals.
When Cu2+ ion is treated with KI, a white precipitate is formed. Explain the reaction with the help of a chemical equation.(Chemistry book)
The final product of treating Cu2+ ions with KI is Cu2I2 white precipitate.
2Cu2+ + 4I− → Cu2I2 (White ppt.) + I2
(CuI2 is formed in this reaction, and because it is unstable, it dissociates into Cu2I2 and I2).
Although fluorine is more electronegative than oxygen, but the ability of oxygen to stabilise higher oxidation states exceeds that of fluorine. Why?
Fluorine is incapable of forming multiple bonds with metals, but oxygen is able to do so. Therefore, oxygen is better than fluorine at stabilizing a higher oxidation state.
Ionisation enthalpies of Ce, Pr and Nd are higher than Th, Pa and U. Why?
Actinoids (Th, Pa, and U) have an incomplete 5f shell, while lanthanoids (Ce, Pr, and Nd) have an incomplete 4f shell.
5f-orbitals will pierce the electron inner core less deeply once they are occupied. Because of this, the 5f-electrons will be better insulated from the nuclear charge than the corresponding lanthanoids’ 4f electrons.
Therefore, The actinoids have available outer electrons for bonding because they are less tightly bound.
Although Zr belongs to 4d and Hf belongs to 5d transition series but it is quite difficult to separate them. Why?
Because of lanthanoid contraction, Zr and Hf separation is quite difficult. They are nearly the same size (Zr = 160 pm and Hf = 159 pm) and share similar chemical properties as a result of lanthanoid contraction. It is difficult to separate them using chemical methods because of this.
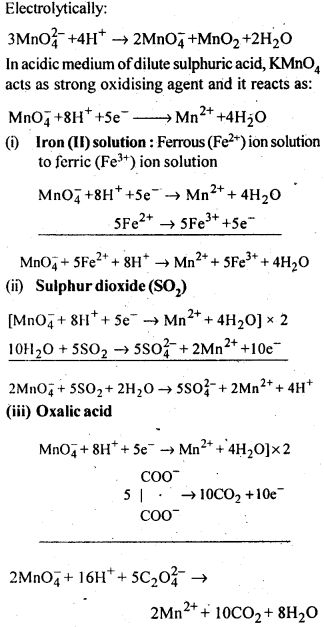
Explain why does colour of KMnO4, disappear when oxalic acid is added to its solution in acidic medium.
An oxidizing agent is KMnO4. Oxalic acid is oxidized to CO2 and then transforms into the colorless Mn2+ ion.
Although +3 oxidation states is the characteristic oxidation state of lanthanides but cerium shows +4 oxidation state also. Why?
Ce3+ obtains a stable 4f0 electronic configuration following the loss of one additional electron. Cerium exhibits a +4 oxidation state, despite the fact that the +3 oxidation state is the typical lanthanoids oxidation state.
The second and third rows of transition elements resemble each other much more than they resemble the first row. Explain why?
The atomic radii of the second and third-row transition elements are nearly equal because of lanthanoid contraction. As a result, they are far more alike than first-row elements.
The halides of transition elements become more covalent with increasing oxidation state of the metal. Why?
The size of the transition element ion decreases as it reaches a higher oxidation state. Fajan’s rule suggests that a bond’s covalent nature increases as the size of the metal ions decreases. As a result, as the metal’s oxidation state increases, the halides of transition elements get more covalent.
Reactivity of transition elements decreases almost regularly from Sc to Cu. Explain.
Because the ionization enthalpy increases regularly, the reactivity of transition elements decreases almost regularly from Sc to Cu.
E0 of Cu is + 0.34V while that of Zn is – 0.76V. Explain.
Cu(s) has a high ionization enthalpy that is not balanced by its hydration enthalpy to become Cu2+ (aq). In the case of zinc, however, a stable 3d10 configuration is obtained following the removal of electrons from the 4s-orbital. As a result, Cu’s E0 value is + 0.34V and Zn’s is – 0.76V.
While filling up of electrons in the atomic orbitals, the 4s orbital is filled before the 3d orbital but reverse happens during the ionisation of the atom. Explain why?
The filling of atomic orbitals occurs in increasing order of energy. According to the (n+l) rule, the 3d orbital is filled after the 4s orbital because its energy exceeds that of the latter. However, electrons in the outermost orbital are lost during ionization. In this instance, electrons from orbital 4s will be ionized first since it is the outermost orbital.
Mention the type of compounds formed when small atoms like H, C and N get trapped inside the crystal lattice of transition metals. Also give physical and Chemical characteristics of these compounds.
Interstitial compounds are those created when minuscule H, C, or N atoms become stuck inside the metal crystal lattice. Several interstitial compounds are formed by the transition metals. Interstitial compounds are created when transition metals react with elements like hydrogen, carbon, nitrogen, boron, etc.
Interstitial compounds’ chemical and physical characteristics
- Compared to the parent transition metals, these compounds have melting points that are significantly higher.
- They have conductivity that is comparable to that of their parent metal.
- These substances are inert chemically.
- These substances are extremely hard. Certain borides have hardnesses that are similar to diamonds.
Check our 12th Chemistry exam solution of chapter 1 click here.
Check our 12th Chemistry exam solution of chapter 2 click here.
Check our 12th Chemistry exam solution of chapter 3 click here.
Check our 12th Chemistry exam solution of chapter 4 click here.
Check our 12th Chemistry exam solution of chapter 5 click here.
Check our 12th Chemistry exam solution of chapter 6 click here.
Check our 12th Chemistry exam solution of chapter 7 click here.
Want some good health click here.